From T-Cells to Exercise: Decoding Immune System Regulation
Discover how metabolism fuels immune system regulation, from T-cells to diet and exercise. Unlock your immune health.

Why Your Immune System Needs More Than Just Activation

Immune system regulation is the process by which your body controls when, where, and how strongly your immune cells respond to threats—and it depends entirely on metabolism, the hidden fuel system that powers every immune response.
Quick Answer: How Immune System Regulation Works
- Metabolic switching - Immune cells shift between energy-saving and energy-spending states based on need
- Fuel selection - Different immune responses use different fuel sources (glucose, fats, or amino acids)
- Cell-specific needs - Each immune cell type has unique metabolic requirements for proper function
- Balance is key - Too much or too little metabolic activity can lead to chronic inflammation or immune dysfunction
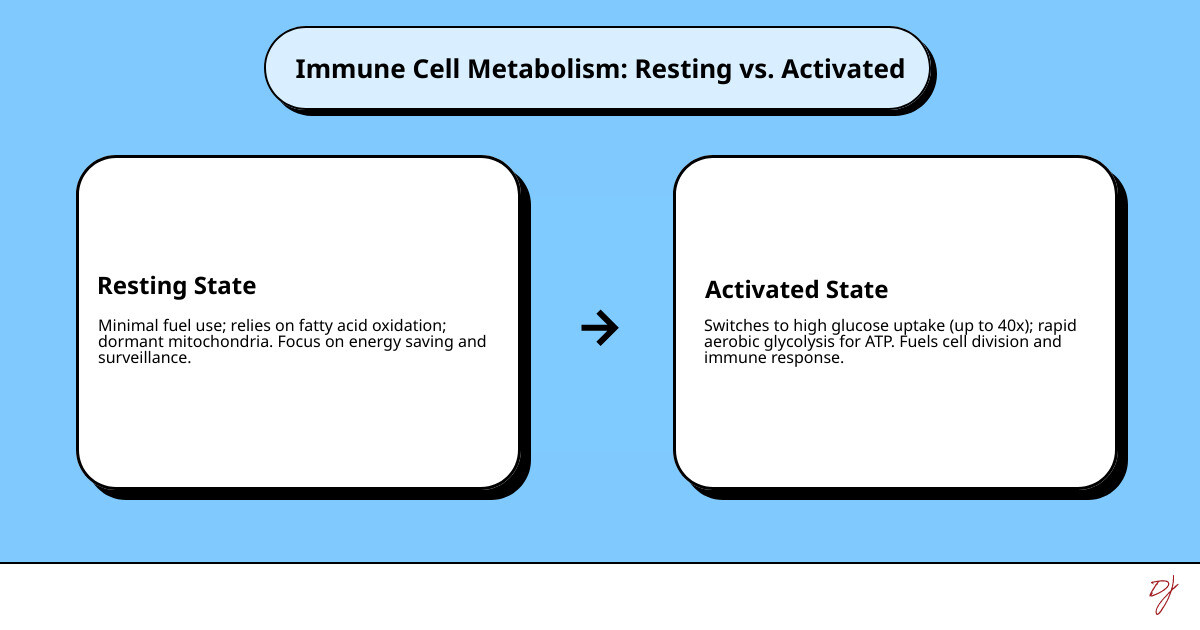
Your immune cells aren't just waiting around for the next infection. They're constantly making decisions about how to spend their energy. A resting T cell uses minimal fuel, like a car idling in park. But when it detects a threat, it switches gears completely—ramping up glucose consumption by up to 40 times to fuel rapid division and attack.
This metabolic reprogramming is called immunometabolism, and it's the difference between an immune system that protects you and one that attacks you.
When this fuel system breaks down, immune cells can get stuck in the wrong gear. Some chronic conditions—like persistent food allergies, long COVID, and autoimmune diseases—involve immune cells that are metabolically dysregulated. They're either burning through fuel too quickly (causing inflammation) or running on empty (failing to respond when needed).
I'm Dr. Doug Jones, a board-certified immunologist who has spent over a decade helping patients with complex immune conditions understand the science behind their symptoms. Through my work at GAIN—Global Allergy Immune Network—I've seen how understanding immune system regulation at the metabolic level can transform how we approach chronic immune challenges.
The Engine of Immunity: How Metabolism Fuels Your Immune Cells
Imagine your immune system as a highly specialized army. Each cell type has a distinct role, from the rapid deployment of frontline defenders to the precise targeting of long-term memory cells. What fuels this army, allowing it to adapt and respond effectively? The answer lies in its metabolism. Immunometabolism is the study of how metabolic pathways regulate immune cell function and differentiation, revealing how deeply intertwined our body's energy production is with its defense mechanisms.
The role of immunometabolism is central to both innate and adaptive immunity. Innate immunity, our body's first line of defense, needs to react quickly and powerfully. Adaptive immunity, on the other hand, requires sustained responses, memory formation, and finely tuned differentiation. These different demands are met by distinct metabolic strategies.
Immune cells are masters of metabolic checkpoints, switching between energy-saving (quiescent) and energy-spending (activated) states. When resting, immune cells, much like a car waiting at a stoplight, primarily use oxidative phosphorylation (OXPHOS). This process, which occurs in the mitochondria, is highly efficient, producing a large amount of ATP (cellular energy) from a relatively small amount of fuel, but it's slower. However, upon activation, these cells undergo a dramatic metabolic shift. They often switch to rapid glycolysis, even in the presence of oxygen, prioritizing speed and the production of building blocks over sheer energy efficiency.
For a deeper dive into the fundamental workings of our body's defense system, you can explore more info about immunity. The mitochondria, often called the "powerhouses of the cell," are not just about energy production; they are also crucial signaling hubs, influencing cell fate, inflammation, and differentiation.
Metabolic Reprogramming in First Responders
Our innate immune cells—the "first responders" of the immune system—are excellent examples of metabolic flexibility. Macrophages, dendritic cells, and neutrophils all use metabolic reprogramming to fulfill their critical roles.
- Macrophages: These versatile cells act as scavengers, engulfing pathogens and cellular debris. When activated to become pro-inflammatory (M1 phenotype), they dramatically increase glycolysis, providing the rapid energy needed for phagocytosis, cytokine production, and antimicrobial activity. Conversely, anti-inflammatory or tissue-repairing macrophages (M2 phenotype) shift towards oxidative phosphorylation and fatty acid oxidation, pathways that support sustained activity and tissue remodeling.
- Dendritic cells: As crucial antigen-presenting cells, dendritic cells bridge the gap between innate and adaptive immunity. Upon activation, they also undergo glycolytic reprogramming, which is essential for their maturation, migration to lymph nodes, and efficient presentation of antigens to T cells. This metabolic shift supports their ability to initiate a robust adaptive immune response.
- Neutrophils: These are the most abundant white blood cells and are rapidly deployed to sites of infection or injury. Neutrophils rely heavily on glycolysis for their immediate, powerful, but short-lived responses. Their energy demands for functions like phagocytosis—the process of engulfing and destroying pathogens—are immense. This rapid glucose consumption fuels their movement, the generation of reactive oxygen species (ROS) to kill microbes, and the formation of neutrophil extracellular traps (NETs), which are web-like structures that trap and neutralize pathogens. Their metabolic pathways are geared for immediate action rather than long-term endurance, which contributes to their critical role in acute inflammation and defense against bacterial infections.
The Warburg Effect in Immunity
One of the most fascinating metabolic shifts in activated immune cells is the Warburg effect. Historically observed in cancer cells, it describes a phenomenon where cells dramatically increase glucose uptake and convert most of it to lactate, even when oxygen is plentiful. This process, known as aerobic glycolysis, is less efficient at producing ATP per glucose molecule than oxidative phosphorylation, yet it is favored by many activated immune cells.
Why would immune cells choose speed over efficiency? The answer lies in their urgent needs. First, aerobic glycolysis provides energy much faster than OXPHOS, allowing for rapid proliferation and immediate effector functions like cytokine production. Second, and perhaps just as importantly, this pathway provides crucial metabolic intermediates. These are the "building blocks" needed for the rapid synthesis of macromolecules like nucleotides (for DNA and RNA), lipids (for new cell membranes), and amino acids—all essential for the massive cellular growth and division that occurs during an immune response. For example, when a T cell encounters a pathogen, it needs to divide rapidly to create an army of effector cells, and the Warburg effect provides the necessary raw materials for this expansion.
A Tale of Two Systems: Metabolism in Adaptive Immunity
While innate immunity relies on quick, broad responses, adaptive immunity is characterized by its specificity, memory, and the intricate coordination of T cells and B cells. Metabolism plays a sophisticated role in shaping the activation, differentiation, and long-term memory functions of these adaptive immune cells.
The Metabolic Life of a T Cell
T cells are central players in adaptive immunity, responsible for directly killing infected cells (CD8+ T cells) or orchestrating other immune cells (CD4+ T helper cells). Their metabolic requirements are highly dynamic and depend on their activation state and differentiation pathway.
Upon activation, naive T cells undergo a metabolic change, shifting towards increased glycolysis and glutaminolysis to support rapid proliferation and effector functions. However, as they differentiate into various subsets, their metabolic preferences diverge:
- Glycolysis: Activated T cells, particularly effector CD8+ T cells (which kill infected cells) and certain CD4+ helper subsets like Th1 and Th17 cells (involved in fighting intracellular pathogens and autoimmune responses), heavily rely on glycolysis. This rapid energy production fuels their immediate cytotoxic or cytokine-producing functions.
- Oxidative Phosphorylation (OXPHOS): As T cells transition from effector to memory cells, they shift their metabolism towards OXPHOS and fatty acid oxidation (FAO). This metabolic switch is crucial for their survival and longevity, allowing them to efficiently generate ATP for long-term maintenance and readiness for future encounters.
- Fatty Acid Oxidation (FAO): Memory T cells and regulatory T cells (Tregs) often depend on FAO. For memory cells, FAO provides a sustained energy source for their long lifespan. For Tregs, which suppress immune responses, FAO is critical for maintaining their suppressive function and stability in various tissue environments.
- Amino Acid Metabolism: Glutamine metabolism, a type of amino acid metabolism, also plays a significant role, particularly in rapidly proliferating T cells, providing both energy and building blocks for macromolecule synthesis.
To illustrate these differences, consider the varied metabolic landscapes of T cell subsets:
| T Cell Subset | Primary Metabolic Pathways | Key Role |
|---|---|---|
| Th1 | Glycolysis, Glutaminolysis | Fight intracellular pathogens, promote inflammation |
| Th2 | Glycolysis, OXPHOS | Fight extracellular parasites, allergic reactions |
| Th17 | Glycolysis, Glutaminolysis | Fight extracellular bacteria/fungi, involved in autoimmunity |
| Treg | Oxidative Phosphorylation (OXPHOS), Fatty Acid Oxidation | Suppress immune responses, maintain tolerance |
| CD8+ Effector | Glycolysis, Glutaminolysis | Kill infected cells, rapid proliferation |
| CD8+ Memory | Oxidative Phosphorylation (OXPHOS), Fatty Acid Oxidation | Long-term protection, rapid recall upon re-encounter |
Fueling Antibody Factories: B Cell Immunometabolism
B cells are the masterminds behind humoral immunity, producing antibodies that neutralize pathogens and toxins. Like T cells, B cell activation and differentiation are profoundly influenced by their metabolic state.
Upon encountering an antigen, naive B cells are activated and undergo clonal expansion and differentiation into antibody-producing plasma cells or memory B cells. This process requires a significant metabolic overhaul. Activated B cells increase glucose uptake and shift towards glycolysis and glutaminolysis to support their rapid proliferation.
Plasma cells, the "antibody factories," are metabolic powerhouses. Their primary role is to synthesize and secrete massive quantities of antibodies, which are complex proteins. This high-volume protein production demands enormous amounts of energy and biosynthetic precursors. Consequently, plasma cells exhibit extremely high rates of glycolysis, OXPHOS, and amino acid metabolism to fuel their endoplasmic reticulum expansion and protein synthesis machinery. Without this robust metabolic support, effective antibody production and sustained humoral immunity would be impossible.
Understanding this metabolic dance is particularly relevant when considering conditions like allergies, where the immune response is misdirected. You can find more info about the immune response in allergies to see how these intricate processes can sometimes go awry.
When Good Fuel Goes Bad: Metabolism's Role in Immune Dysfunction
While healthy immune cells expertly switch their metabolic gears, sometimes this finely tuned system goes awry. When metabolic pathways become dysfunctional, it can lead to chronic inflammation and immune disorders. Imagine our immune cells constantly burning fuel in an inefficient, pro-inflammatory manner. This sustained metabolic activity, often driven by altered nutrient signaling and environmental factors, can contribute significantly to the pathogenesis of various diseases.
Understanding Dysfunctional Immune System Regulation in Autoimmunity
Metabolic changes in immune cells are increasingly recognized as key contributors to the development and progression of inflammatory and autoimmune diseases. In these conditions, the immune system regulation breaks down, and immune cells mistakenly attack the body's own tissues.
- Metabolic signatures in autoimmune diseases: Research has uncovered distinct metabolic signatures in immune cells from individuals with autoimmune conditions. For example, T cells in patients with systemic lupus erythematosus (lupus) often exhibit altered glycolysis and mitochondrial function, contributing to their hyperactive state. Similarly, in rheumatoid arthritis, the synovial fluid (joint fluid) is often hypoxic (low oxygen) and nutrient-deprived, pushing immune cells within the joint towards glycolytic metabolism, which in turn fuels the inflammatory cycle.
- Pro-inflammatory cell metabolism: Sustained glycolytic metabolism in cells like M1 macrophages and effector T cells can perpetuate chronic inflammation. This metabolic state supports the continuous production of pro-inflammatory cytokines, creating a vicious cycle of tissue damage.
- Treg cell metabolic defects: Regulatory T cells (Tregs) are crucial for suppressing immune responses and maintaining self-tolerance. Their proper function heavily relies on oxidative phosphorylation and fatty acid oxidation. If these metabolic pathways are impaired in Tregs, their ability to control other immune cells diminishes, potentially allowing autoimmune reactions to flourish.
For those struggling with such conditions, understanding the underlying mechanisms is the first step toward finding solutions. You can find more info about autoimmune disease to learn about approaches to managing these complex challenges.
The Interplay of Environment, Epigenetics, and Metabolism
Our immune cells don't operate in a vacuum; they are profoundly influenced by their surroundings. Environmental factors, including nutrient availability and oxygen levels, directly impact immune cell metabolism and function. Furthermore, these metabolic changes can have lasting effects through epigenetic modifications.
- Nutrient availability: The availability of key nutrients like glucose, amino acids, and fatty acids directly dictates which metabolic pathways immune cells can use. For instance, high glucose concentrations can promote pro-inflammatory phenotypes in certain immune cells, while specific amino acids like tryptophan or arginine can influence T cell differentiation.
- Hypoxia (low oxygen) in inflamed tissues: Inflamed tissues often become hypoxic due to increased cellular activity and compromised blood flow. This low-oxygen environment naturally favors glycolysis, as oxygen is not required for this pathway. This metabolic shift in turn promotes the activation and survival of pro-inflammatory immune cells, further exacerbating the inflammatory response.
- How metabolic byproducts alter gene expression (epigenetics): This is where the story gets really intricate. Metabolites produced during cellular metabolism are not just energy sources; they also act as cofactors or substrates for enzymes that regulate gene expression through epigenetic modifications. For example, acetyl-CoA, a product of glucose and fatty acid metabolism, is essential for histone acetylation, which generally "opens up" DNA for gene transcription. Similarly, alpha-ketoglutarate, an intermediate in amino acid metabolism, influences histone demethylation. This direct link means that what an immune cell "eats" can literally change which genes it "reads," thereby dictating its identity and function.
- The impact of diet and lifestyle: This intricate connection highlights how our diet and lifestyle choices can profoundly influence our immune system's metabolic health and, consequently, its ability to maintain proper immune system regulation.
The Next Frontier: Advancing Immune System Regulation for Health
The profound understanding of immunometabolism is opening exciting new avenues for therapeutic intervention. By targeting the metabolic pathways within immune cells, we can potentially modulate their function, offering new strategies for treating a wide range of immune-related diseases and even enhancing cancer immunotherapies. This represents a significant shift in our approach to immunology, moving beyond simply activating or suppressing immune cells to fine-tuning their internal engines.
Approaches to Supporting Immune System Regulation
Research is actively exploring various approaches to leverage immunometabolism for health benefits:
- Research-based strategies: Scientists are investigating cellular therapies that involve metabolically engineering immune cells to improve their anti-tumor activity or to reduce their pro-inflammatory potential in autoimmune settings. This involves understanding the precise metabolic needs of different immune cell types and then manipulating those pathways.
- Exploring immune cell metabolism in disease: Continual research helps us pinpoint specific metabolic vulnerabilities in diseased immune cells. For instance, identifying a reliance on a particular metabolic pathway in cancer cells or hyperactive immune cells could lead to targeted therapies that disrupt that pathway, effectively "starving" the problematic cells or altering their function.
- Restoring metabolic balance in immune conditions: The ultimate goal is to restore a healthy metabolic balance within immune cells. This could involve using specific drugs (metabolic modulators) that block or improve certain metabolic enzymes, or even nutritional interventions that provide the right metabolic cues to guide immune cells towards a more beneficial state. This approach is particularly promising for complex post-viral syndromes, where immune dysregulation is a central feature. For those experiencing such challenges, understanding the underlying immune shifts is crucial. You can find more info about complex post-viral syndromes to learn more.
The Role of Research and Academic Centers
The development of these cutting-edge therapies and insights into immune system regulation is heavily reliant on ongoing scientific inquiry. Metabolic modulators, drugs that specifically target immune cell metabolism, are a major focus of current research. These agents aim to reprogram immune cells, making them more effective against cancer or less harmful in autoimmune diseases.
The importance of ongoing scientific findings cannot be overstated. Each new findy about how immune cells use energy brings us closer to personalized and more effective treatments. Academic medical centers, such as those that adhere to strict advertising policies to ensure unbiased information, are crucial in driving this innovation and understanding. These institutions, like the Cleveland Clinic, are often at the forefront of basic research and clinical trials, translating complex scientific findies into practical applications that benefit patients.
Frequently Asked Questions about Immunometabolism
How does diet affect immune cell metabolism?
Our diet is the primary source of fuel and building blocks for every cell in our body, including immune cells. Nutrient availability directly impacts immune cell metabolism. For example:
- Glucose: High glucose levels can promote glycolysis, which, as we've seen, can drive pro-inflammatory responses in many immune cells.
- Amino Acids: Specific amino acids like glutamine are crucial for rapidly proliferating immune cells, providing both energy and substrates for macromolecule synthesis.
- Fats: Fatty acids are a key energy source for long-lived, quiescent, or regulatory immune cells, fueling oxidative phosphorylation and promoting cell survival.
What we eat directly influences the metabolic state of our immune cells, impacting their function, differentiation, and overall immune system regulation.
Can exercise change my immune system's metabolism?
Yes, exercise can significantly influence your immune system's metabolism, with both acute and chronic effects.
- Acute effects: During strenuous exercise, immune cells are mobilized, circulating more rapidly throughout the body. This acute stress can temporarily alter their metabolic profiles, preparing them for potential challenges.
- Chronic effects: Regular, moderate exercise can lead to improved metabolic health overall, which in turn benefits immune cell function. It can improve mitochondrial efficiency, promote anti-inflammatory pathways, and support the development of a more robust and balanced immune response. Exercise can help shift immune cells towards metabolic states that favor resolution of inflammation and tissue repair.
What is the difference between immunometabolism and general metabolism?
While general metabolism refers to all the chemical processes that occur within a living organism to maintain life, immunometabolism is a specialized subset focusing specifically on immune cells.
- General metabolism: This encompasses the broader "housekeeping" functions of all cells—how they acquire energy, synthesize molecules, and eliminate waste to survive.
- Immunometabolism: This refers to the dynamic and specialized metabolic programs that immune cells adopt to perform their specific functions. Unlike other cells that might maintain a relatively stable metabolic state, immune cells undergo dramatic and rapid metabolic reprogramming in response to activation, differentiation, and changes in their microenvironment. It's about how immune cells strategically use metabolism to fight infections, resolve inflammation, or even contribute to disease.
Conclusion: A New Understanding of Immune Health
The journey through the intricate world of immunometabolism reveals a profound truth: the efficiency and effectiveness of our immune system regulation are inextricably linked to the metabolic pathways powering our immune cells. From the rapid glycolytic burst of a macrophage responding to a threat to the sustained oxidative phosphorylation of a long-lived memory T cell, cellular energy management is central to every aspect of our immune defense.
This connection between cellular energy and overall immune function is not just a fascinating scientific concept; it holds immense potential for empowering us to understand and support our immune health. By recognizing that immune cells are not static entities but dynamic metabolic machines, we gain a new perspective on chronic immune challenges, from persistent allergies to autoimmune conditions. For complex immune challenges like those pioneered by Dr. Doug Jones, seeking expert guidance that considers these intricate metabolic relationships is key to finding lasting relief through personalized care and education.
As our understanding of immunometabolism deepens, we are better equipped to steer the complexities of immune health and work towards more targeted and effective strategies. To learn more about how we approach these intricate challenges, you can learn more about complex immune conditions.
